Research Interest
The research interests of our laboratory focus on the molecular mechanisms underlying the innate immune response and inflammation during microbial infections. The term inflammation can be traced back to ancient Roman observations of signs such as redness, swelling, heat, pain, and loss of function. Microbial infections lead to host immune responses, including transcriptional changes of inflammatory genes. Although a properly controlled, transient immune response is crucial for host defense during infection, a failure in negative feedback suppression of this response can lead to irreparable tissue damage and may even be fatal. It therefore underscores the importance of understanding how inflammatory responses are fine-tuned to maintain physiological homeostasis after a detrimental stimulus.
What we have done
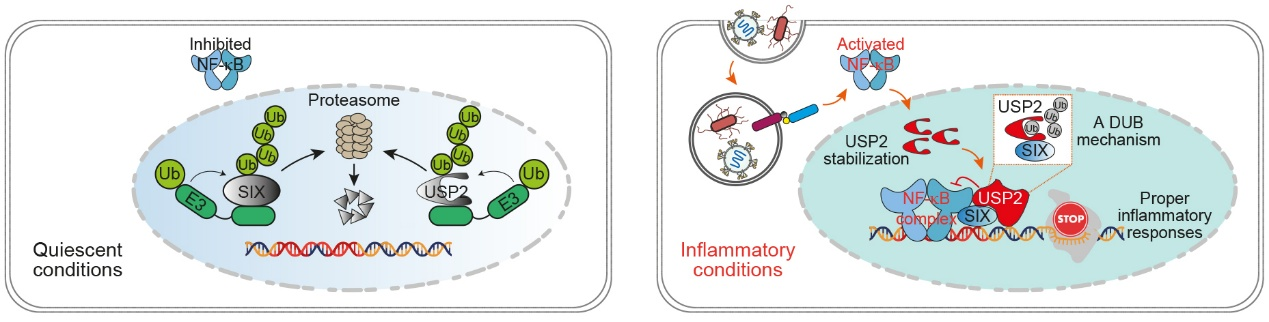
Our previous studies unexpectedly revealed that a family of sine oculis transcription factors (SIX proteins) functions as immune brake via targeting gene promoters in adult immune cells in response to persistent inflammatory stimulation. (Liu et al., Nature, 2019) (Fig. 1). Our recent work has uncovered a previously unrecognized molecular mechanism by which a deubiquitination cascade precisely fine-tunes inflammatory gene transcription in the nucleus (Yi et al., Cell Research, 2025) (Fig. 1). Inflammation is strongly associated with many diseases (e.g., cancer, inflammatory bowel disease [IBD], etc.). We now aim to explore the relationship between inflammation and diseases, as well as the molecular mechanisms underlying this immune checkpoint axis.
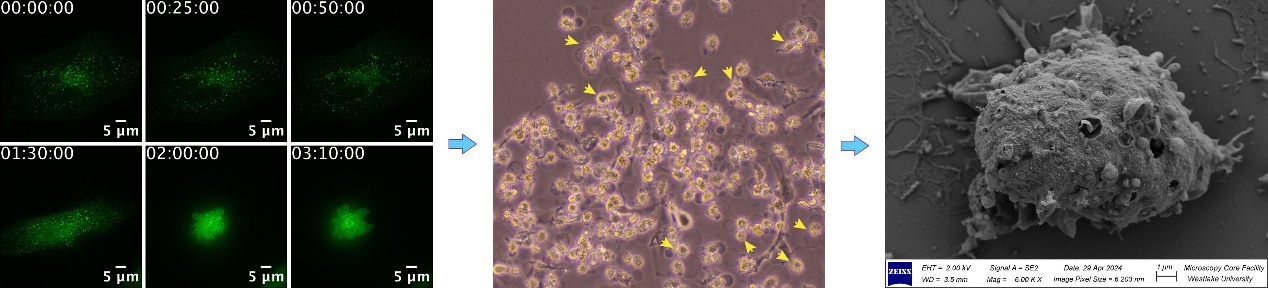
In addition, we are interested in how innate immunity is activated. Our lab has discovered a series of novel components that control innate immunity, cell death, and antibacterial molecular mechanisms (Figs. 2 and 3). Our research will not only expand our understanding of fundamental biomedical problems but also contribute to the development of clinical drugs.